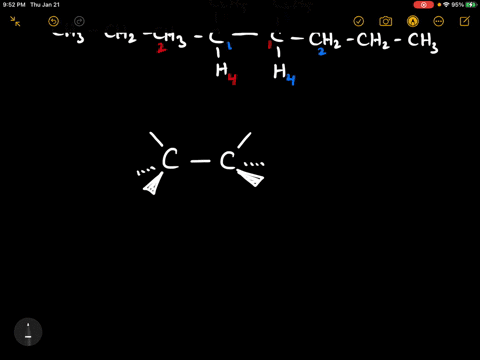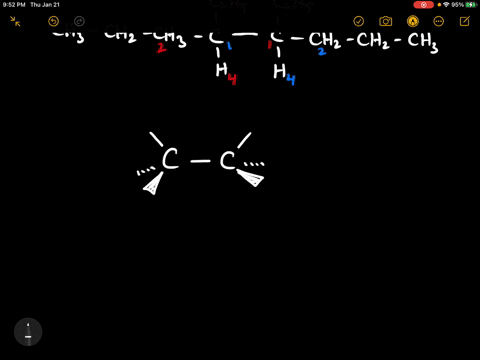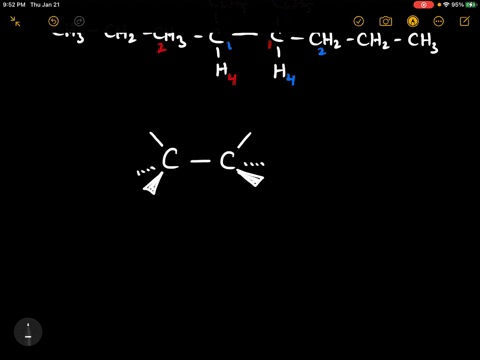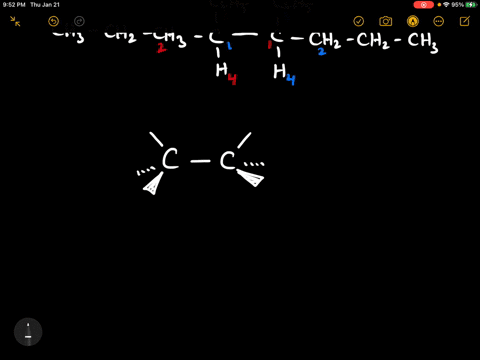
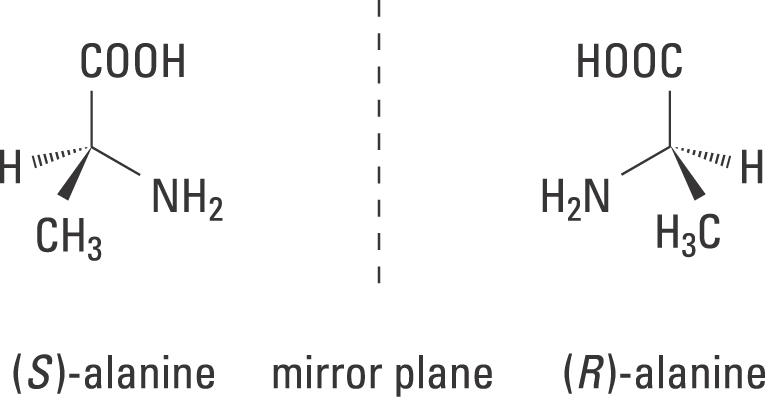
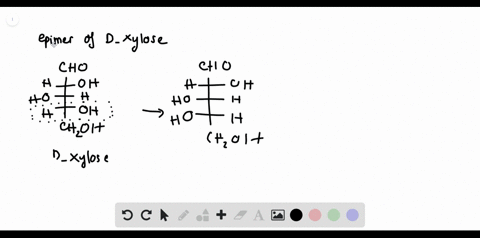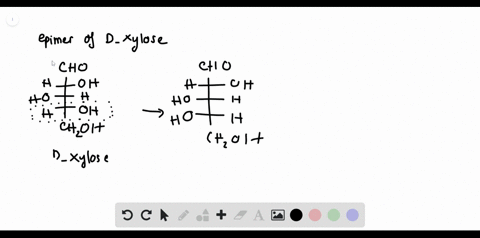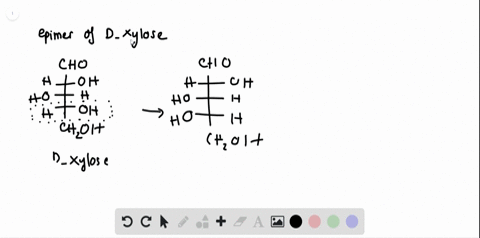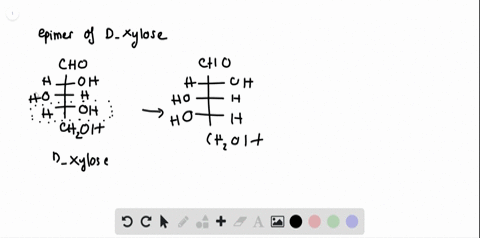
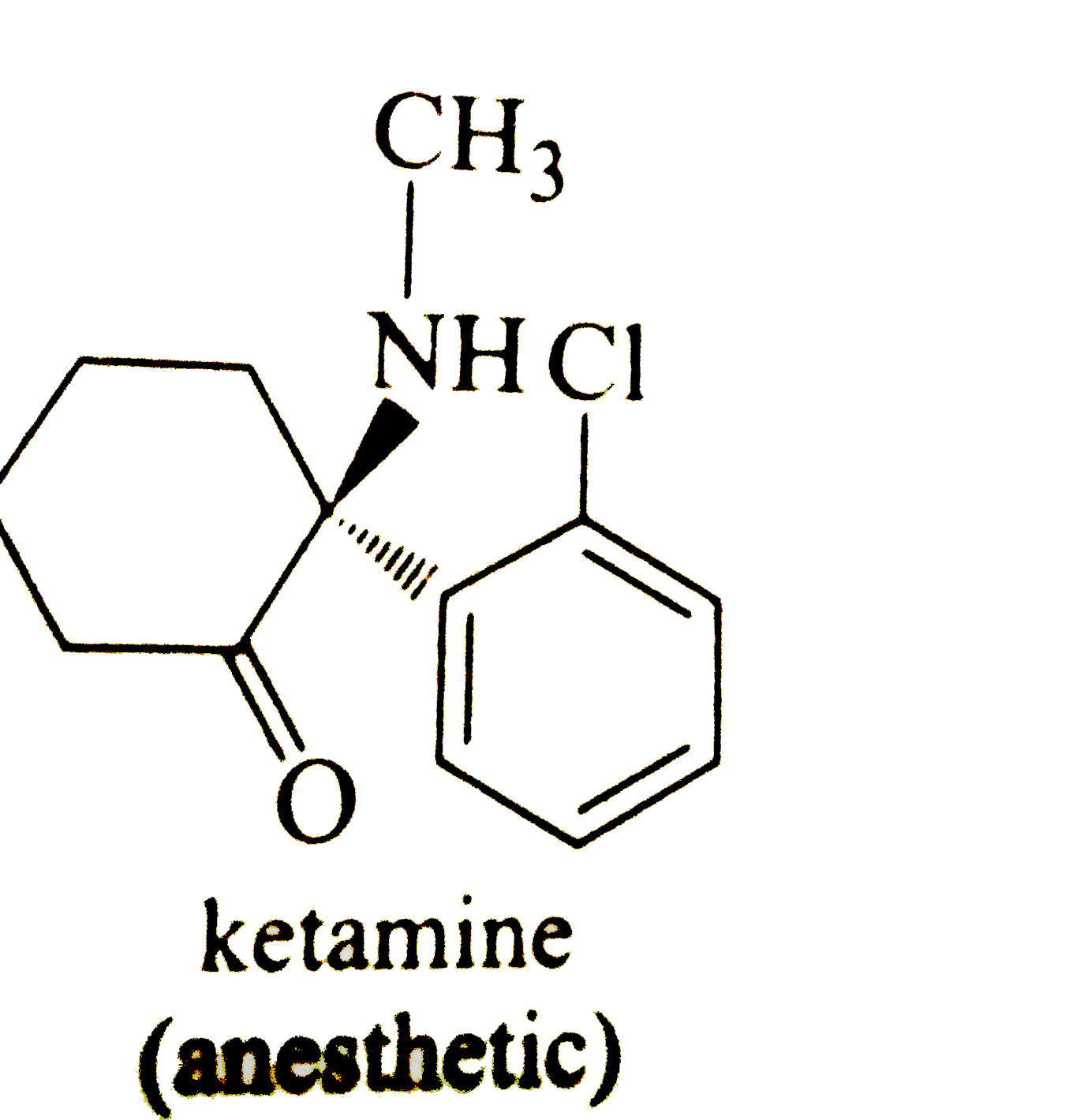
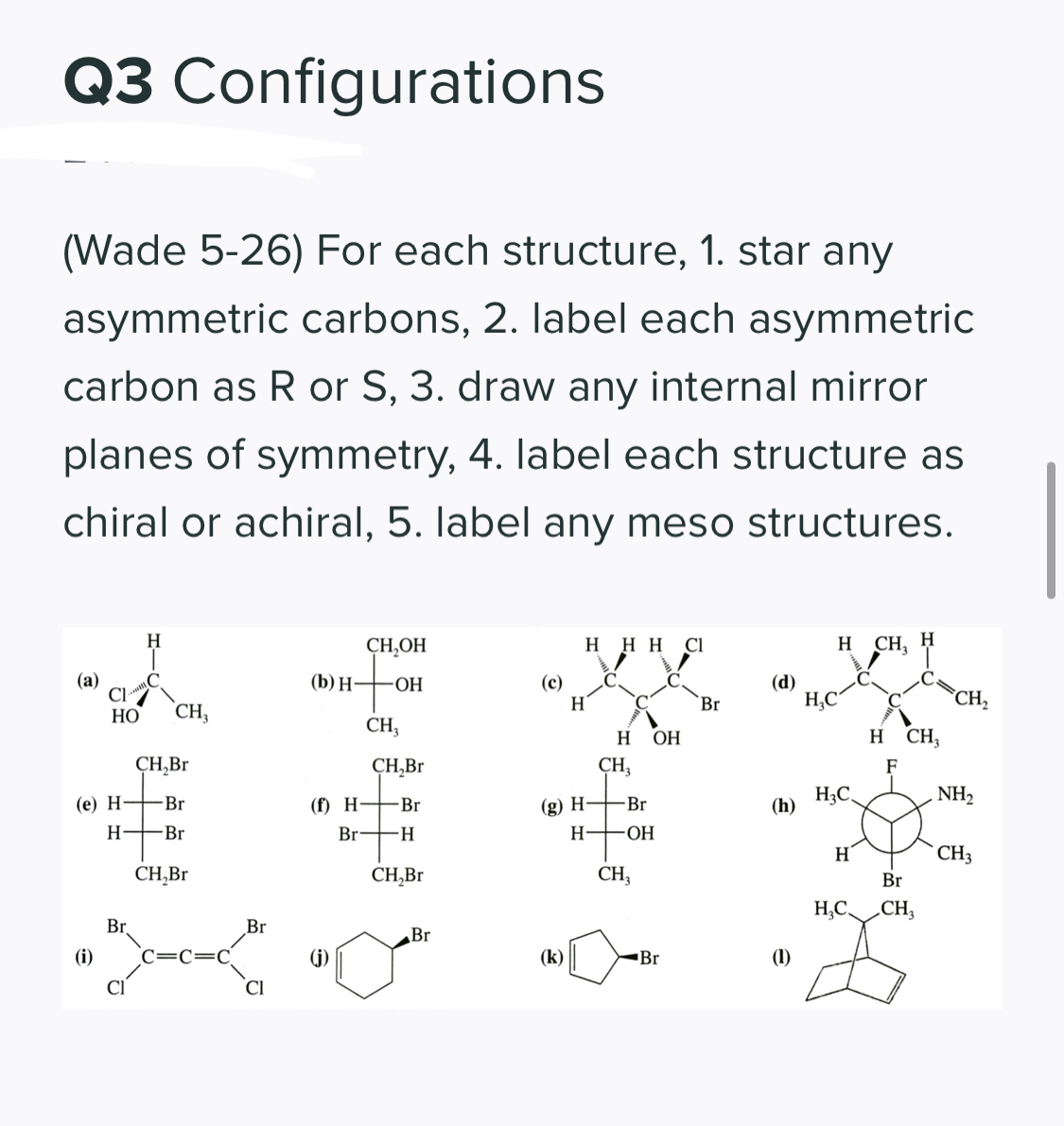
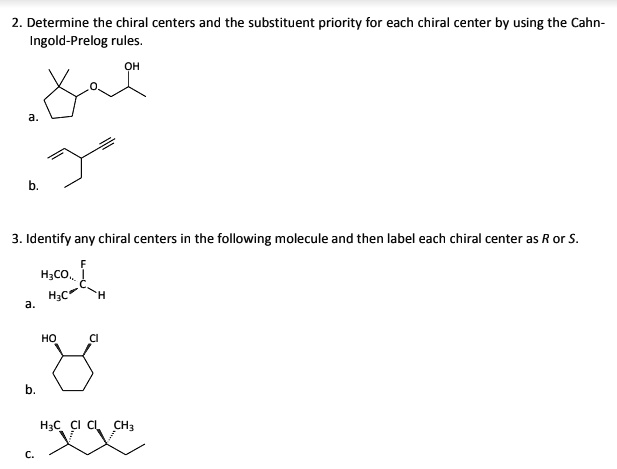
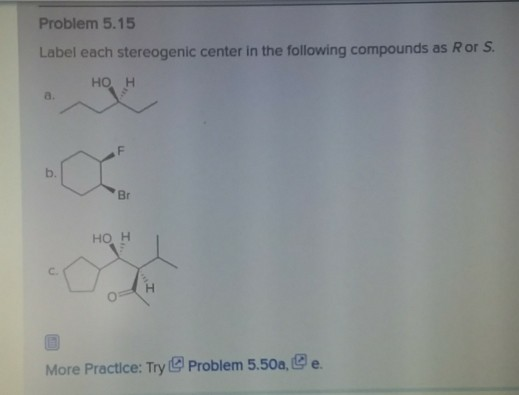
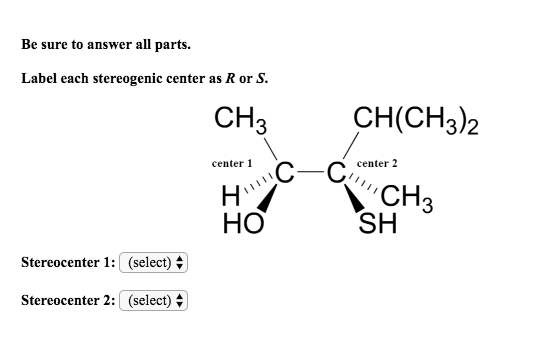
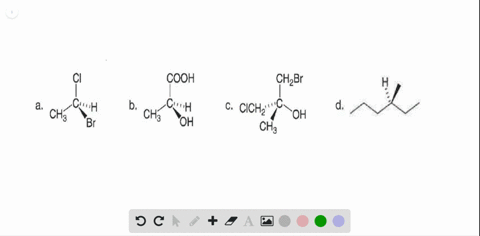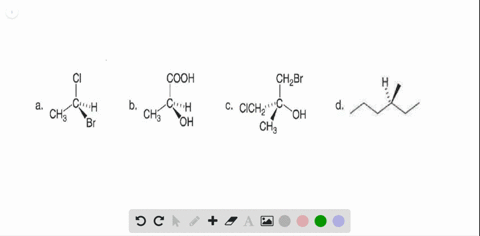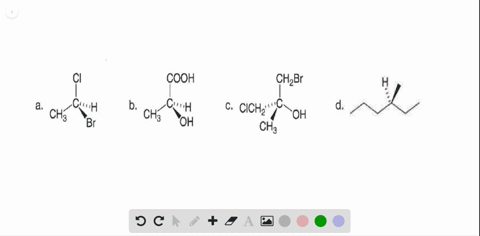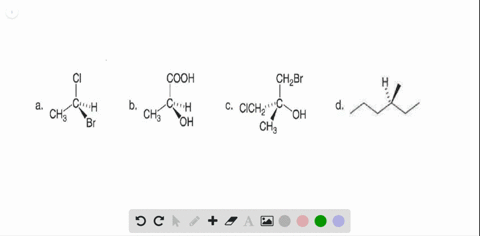
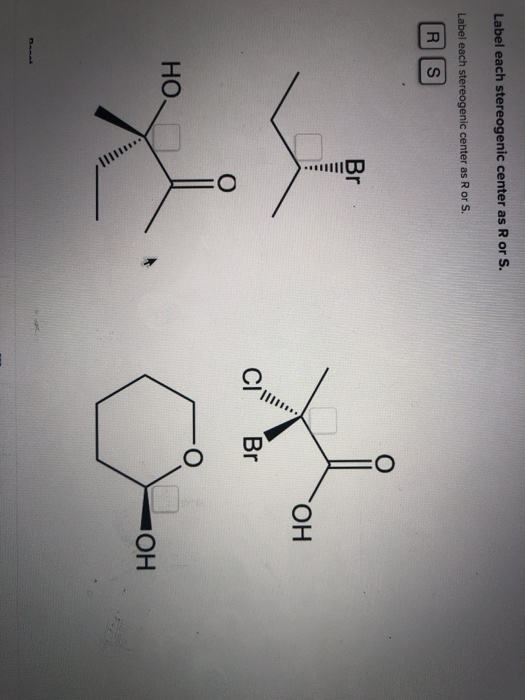
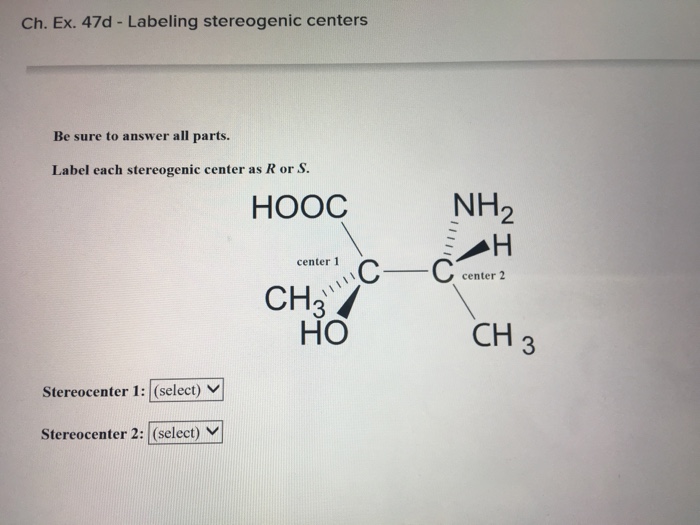
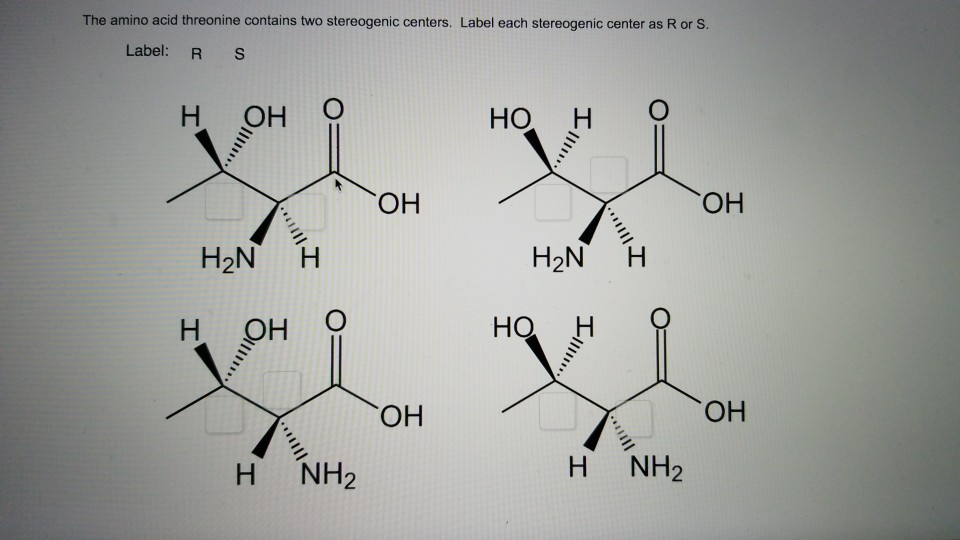
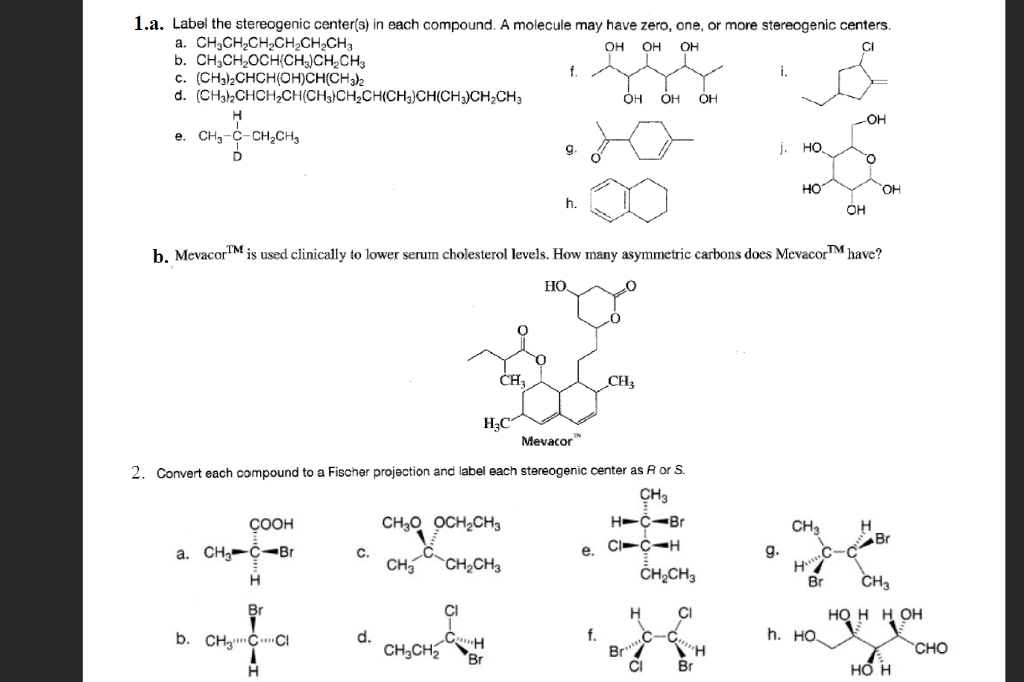
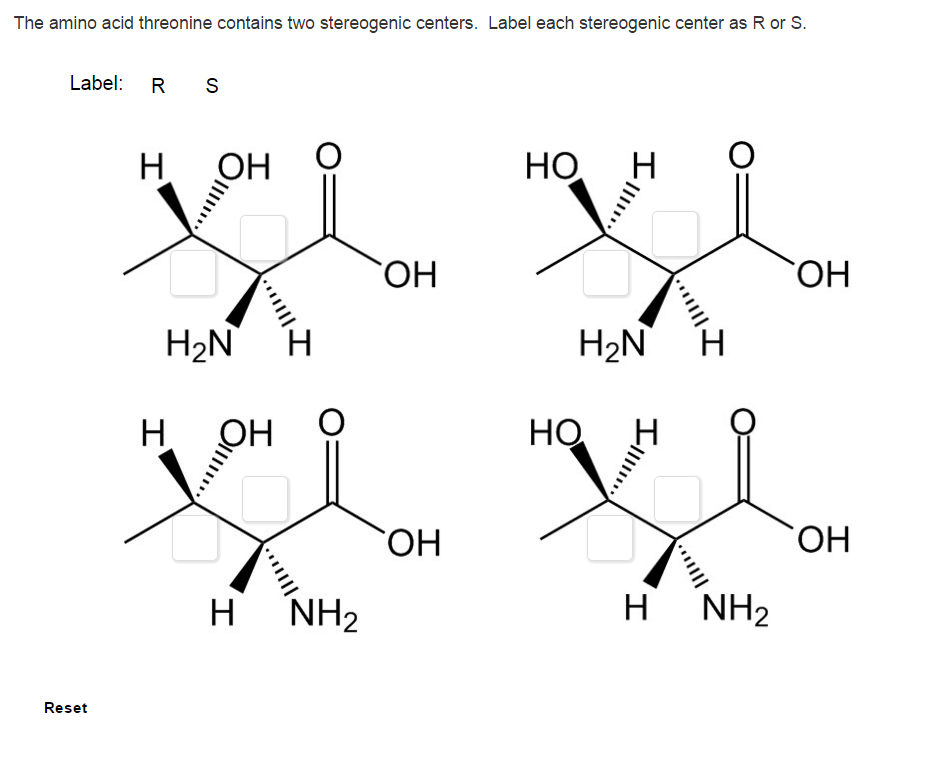
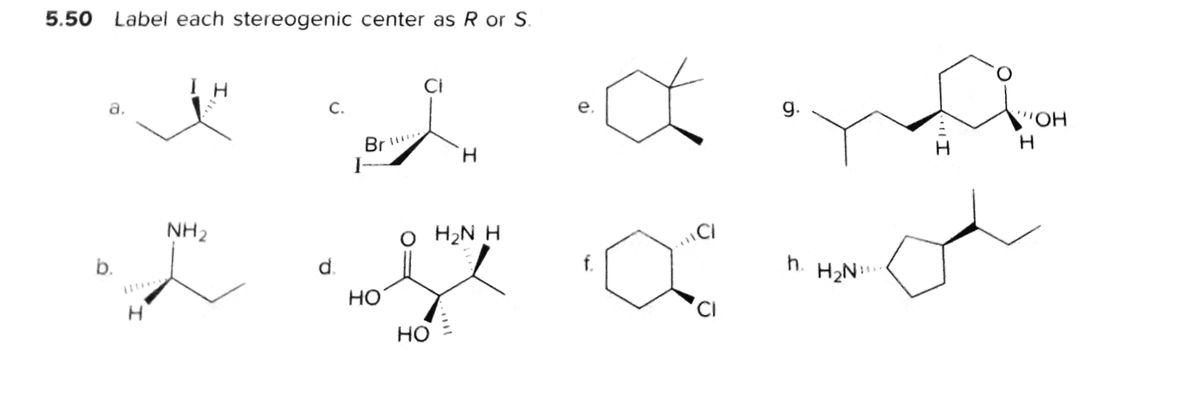
40 label each stereogenic center as r or s
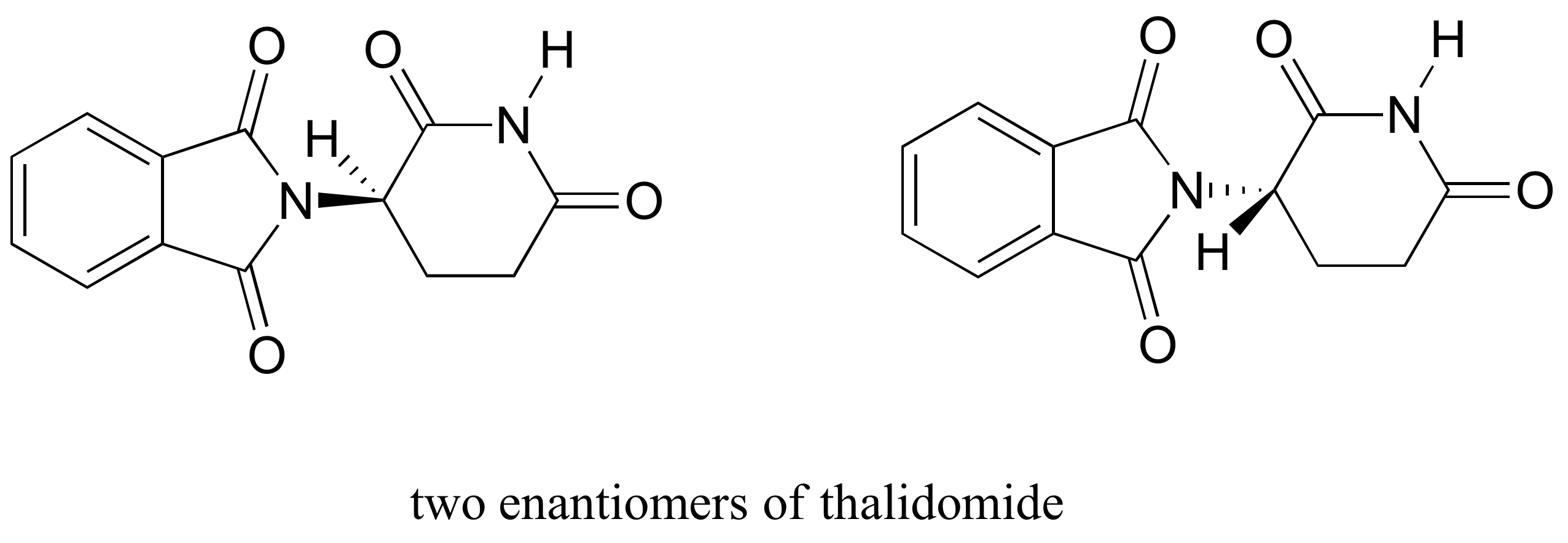
An Iterative Approach Guides Discovery of the FabI Inhibitor Fabimycin ... (A) Debio-1452 is highly potent against S. aureus; Debio-1452-NH3 retains this potency and gains modest activity against many Gram-negative pathogens. (B) The structure-activity relationship (SAR) of Debio-1452 showing regions amenable to substitution (highlighted in green) and those critical for antibacterial activity (highlighted in red). Chemistry Archive | August 09, 2022 | Chegg.com Identify any stereogenic center/s in the above molecule with an asterisk. b. How many 1 answer 13) a. Provide the major organic compound after EACH STEP in the sequence below. 2) \ ( \mathrm {CuBr} \longrightarrow \) b. Show how could you make the above starting compound in two steps starting fro 1 answer Questions are already answered.
Company Curology get st petersburg supercenter store hours and driving directions, buy online, and pick up in-store at 10237 bay pines blvd, st petersburg, fl 33708 or call 727-347-1188 relax and let your skin soak up it's light, gel texture—like putting a cloud on your face, some say a licensed dermatology provider evaluates your skin profile, skin type, and …
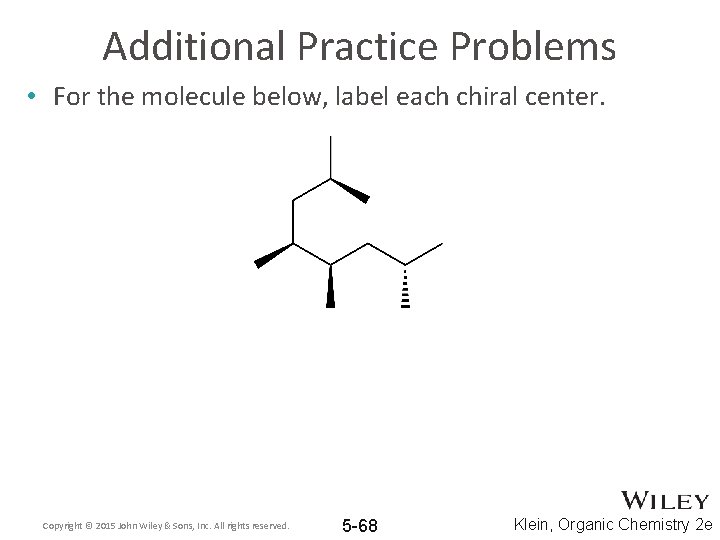
Label each stereogenic center as r or s
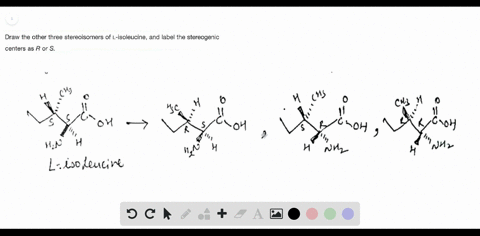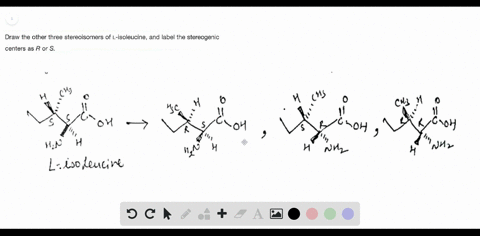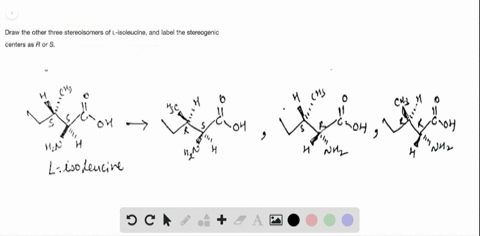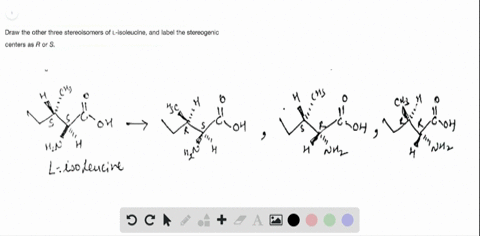
Chemistry Archive | August 08, 2022 | Chegg.com Label each anomer as a or ji. (25 pts) 4. Convert each cyclic monosacchande t. 0 answers pls help 1. Re-write the following reaction sequen 2. ... Predict all product(s). Show stereochemistry at stereogenic centers. 6. Show the product(s) for the following reaction. 1 answer help with 7 and 8 pls 7. What is the structure of the compound with ... Organic Chemistry Bonding Stereochemical Descriptors 1. Draw a Fischer-projection for each of the sugars and assign the corresponding stereochemical descriptor D or L. 2. Assign stereochemical descriptors (R or S) for the labeled stereogenic centers (a-e) in the anticancer drug Taxol: See attached figures. Topicity relationship and isomer relationship problems Company Curology Our mission is simple: to make personalized skincare accessible and convenient for all Get St Petersburg Supercenter store hours and driving directions, buy online, and pick up in-store at 10237 Bay Pines Blvd, St Petersburg, FL 33708 or call 727-347-1188 .
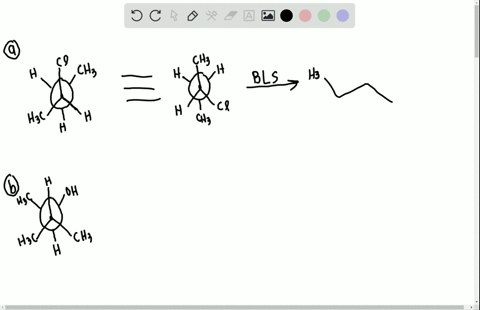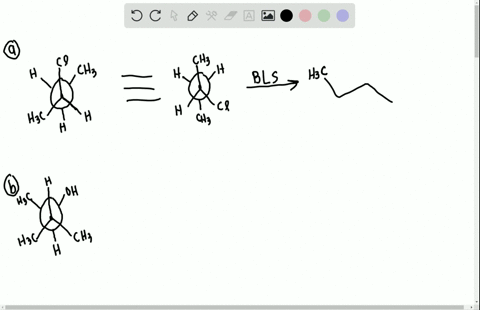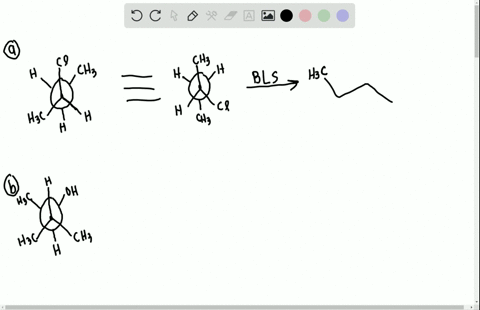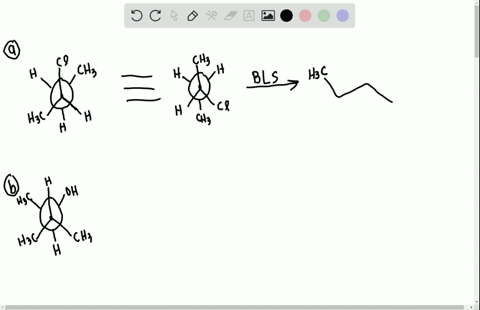
Label each stereogenic center as r or s. Company Curology Our mission is simple: to make personalized skincare accessible and convenient for all Get St Petersburg Supercenter store hours and driving directions, buy online, and pick up in-store at 10237 Bay Pines Blvd, St Petersburg, FL 33708 or call 727-347-1188 . Organic Chemistry Bonding Stereochemical Descriptors 1. Draw a Fischer-projection for each of the sugars and assign the corresponding stereochemical descriptor D or L. 2. Assign stereochemical descriptors (R or S) for the labeled stereogenic centers (a-e) in the anticancer drug Taxol: See attached figures. Topicity relationship and isomer relationship problems Chemistry Archive | August 08, 2022 | Chegg.com Label each anomer as a or ji. (25 pts) 4. Convert each cyclic monosacchande t. 0 answers pls help 1. Re-write the following reaction sequen 2. ... Predict all product(s). Show stereochemistry at stereogenic centers. 6. Show the product(s) for the following reaction. 1 answer help with 7 and 8 pls 7. What is the structure of the compound with ...















Post a Comment for "40 label each stereogenic center as r or s"