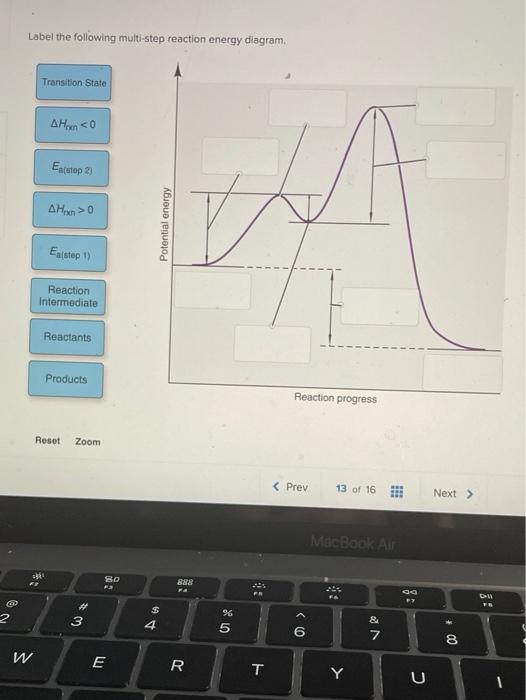
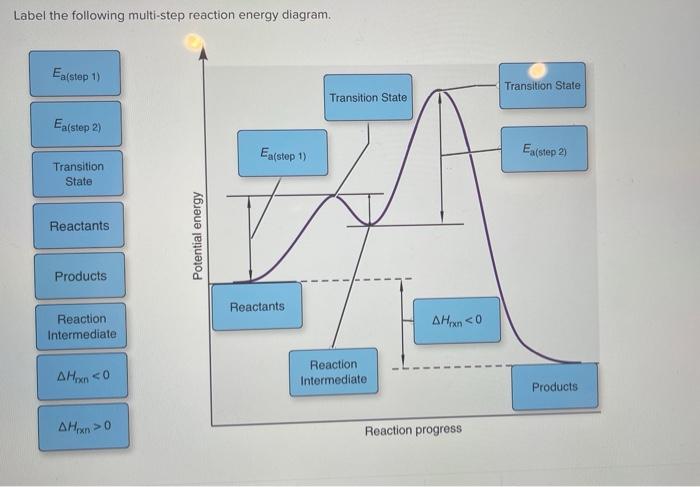
41 label the following multi-step reaction energy diagram
Answered: Step 8 HO. HN. HN | bartleby Q: Answer true or false to the following statements about energy diagrams and reactions. Q.) Thermal… A: In order for a reaction to occur, the collision theory is based on the premise that the reacting… Energy Profiles (Energy Diagrams) Chemistry Tutorial - AUS-e-TUTE An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change, ΔH, is the amount of energy absorbed or released by a chemical reaction. On an energy profile, the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products.
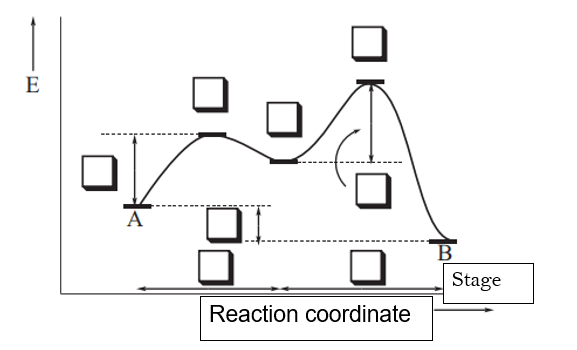
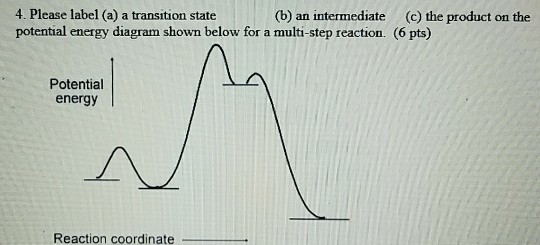
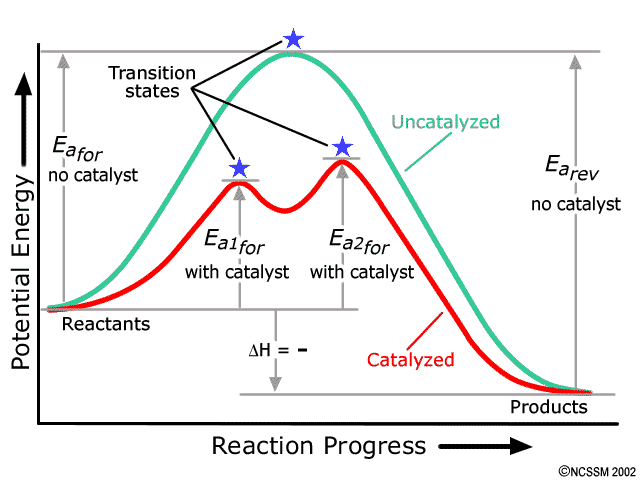
12.7 Catalysis - Chemistry - opentextbc.ca This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction path with a lower activation energy. As shown, the catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two ...
Label the following multi-step reaction energy diagram
Solved Label the following multi-step reaction energy | Chegg.com See the answer See the answer done loading. Label the following multi-step reaction diagram. Show transcribed image text. Solved Label the following reaction energy diagram for a - Chegg Question: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Transition State Transition State Transition intermediate Reaction Intermediate Transition State Transition Uncatalyzed Transition State Reactants Products Catalyzed Ea (fwd) no catalyst Uncatalyzed AHxn < 0 Potential energy Ea (rev) no catalyst Ea ... How does the energy level diagram show this reaction is exothermic? Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
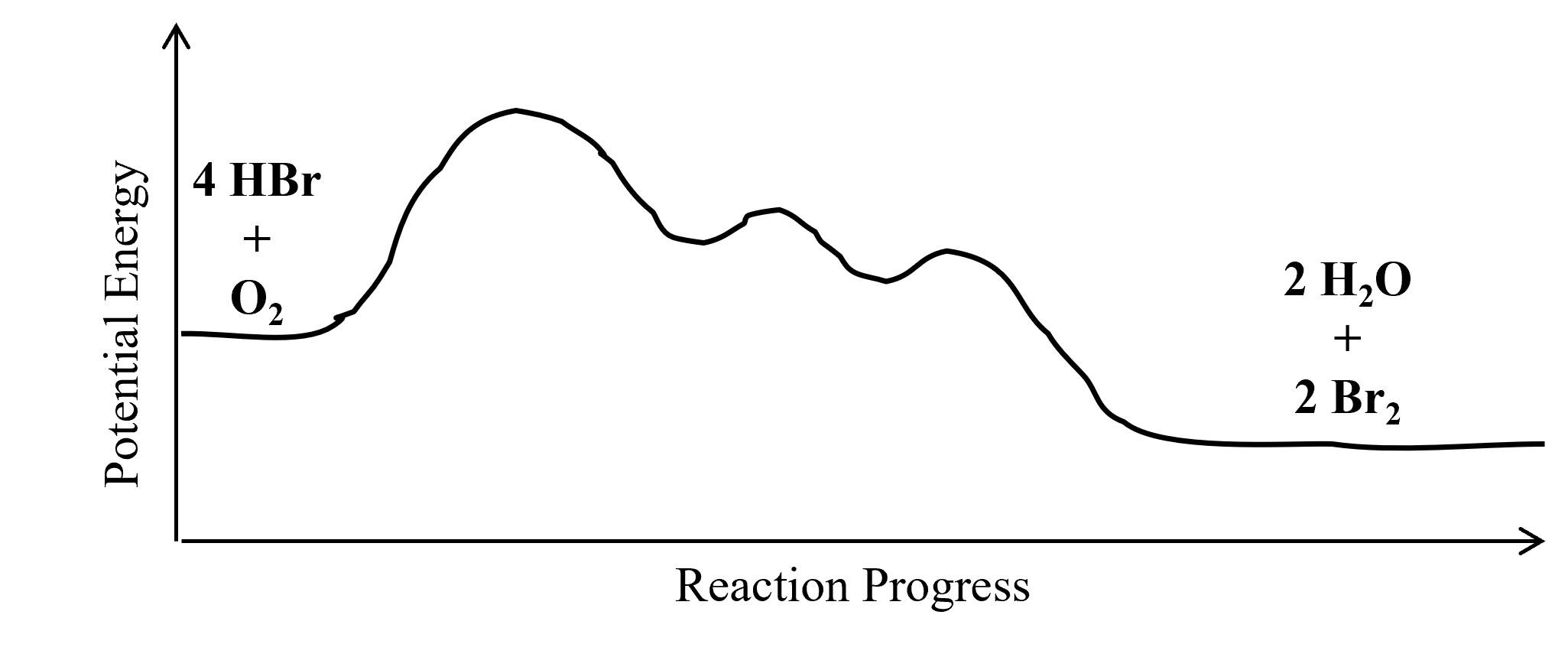
Label the following multi-step reaction energy diagram. Reaction Mechanisms - Chem1 The gas-phase formation of HI from its elements was long thought to be a simple bimolecular combination of H 2 and I 2, but it was later found that under certain conditions, it followes a more complicated rate law. 2 Multi-step (consecutive) reactions Mechanisms in which one elementary step is followed by another are very common. step 1: A + B → Q Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... Consider the following mass distribution, where x- and y-coordinates ... What is an energy level diagram? - BYJUS Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy levels is as follows: Multistep Reactions - Softschools.com N 2 O 2 (g) + O 2 → 2NO 2 (g) (fast) Which is the most likely rate law for the reaction: k [NO] 2, or k [NO] 2 [O 2] The answer is k [NO] 2, since 2 molecules of NO (and no O 2) are involved in the reaction prior to the slow step. To link to this Multistep Reactions page, copy the following code to your site: Multistep Reactions.
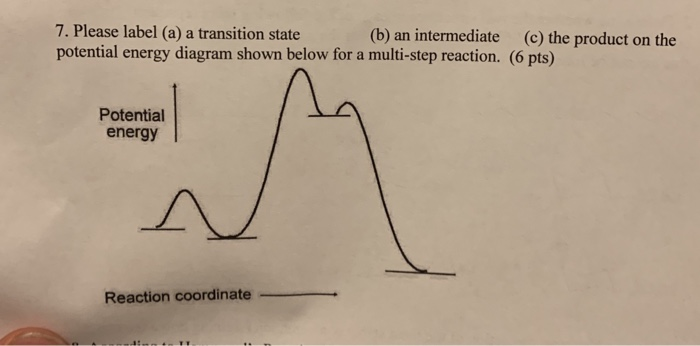
PDF Rates, Temperature and Potential Energy Diagrams Worksheet Draw a potential energy (E p ) diagram for a reaction in which ∆H = 80 kJ/mol and E a = +28kJ/mol. Label the axes, activation energy, ∆H, site of the activated complex, reactants and products. 3. Using the potential energy diagrams for an endothermic and exothermic reaction shown, choose the letter that best fits each statement. PDF 5.12 Spring 2003 Review Session: Exam #2 - MIT OpenCourseWare D. Reaction-Energy Diagrams 1. Thermodynamic Control 2. Kinetic Control 3. Hammond Postulate 4. Multi-Step Reactions 5. Chlorination of Methane E. Chlorination of Propane 1. Inequivalent Hydrogens (1°,2°,3°) 2. Relative Reactivity 3. Selectivity F. Bromination of Propane 1. Answered: Reaction progress Potential Energy | bartleby Q: The diagram below shows the energy profile for a reaction. D В E C A Reaction progress Species… D В E C A Reaction progress Species… A: transition state is a very short lived state of atoms ,in the transition state , Energy of atoms… 12.6 Reaction Mechanisms - Chemistry The reaction mechanism (or reaction path) is the process, or pathway, by which a reaction occurs. A chemical reaction usually occurs in steps, although it may not always be obvious to an observer. The decomposition of ozone, for example, appears to follow a mechanism with two steps:
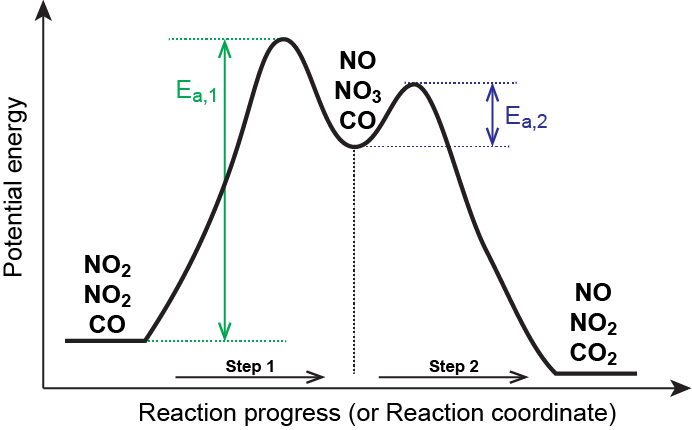
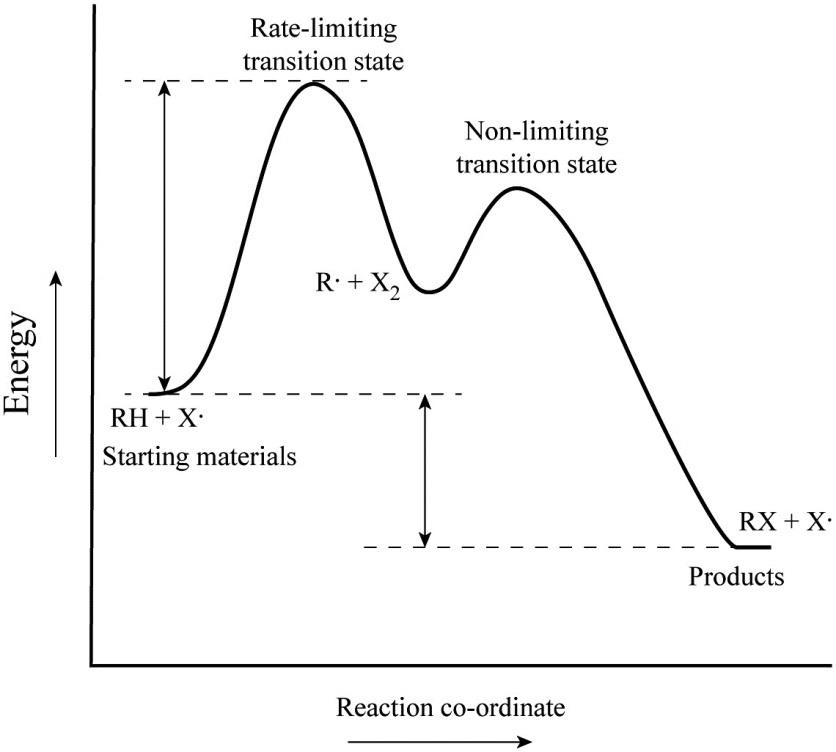
Solved Label the following multi-step reaction energy - Chegg Question: Label the following multi-step reaction energy diagram AHn> 0 Reaction Intermediate Ealstep 2) Products Ea (step 1) Reactants Reaction progress This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (11 ratings) Reaction mechanism and rate law (article) | Khan Academy Summary. A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. Reaction Mechanisms | Boundless Chemistry | | Course Hero The sum of each elementary step in a reaction mechanism must yield the overall reaction equation. The rate law of the rate-determining step must agree with the experimentally determined rate law. The rate-determining step is the slowest step in a reaction mechanism. Because it is the slowest, it determines the rate of the overall reaction. Solved Label the following multi-step reaction energy | Chegg.com Question: Label the following multi-step reaction energy diagram. Eatstep 1) Transition State Transition State Eastep 2) Eastep 1) Ea (step 2) Transition State Reactants Potential energy Products Reactants Reaction Intermediate ΔΗκη <0 AH 0 Reaction Intermediate Products ΔΗρκιν >0 Reaction progress This problem has been solved! See the answer
Energy level diagrams - Why are there energy changes in ... - BBC Bitesize reaction. This is because energy is taken in from the surroundings. An upwards arrow shows that energy is taken in Question. Look at the energy level diagrams below.
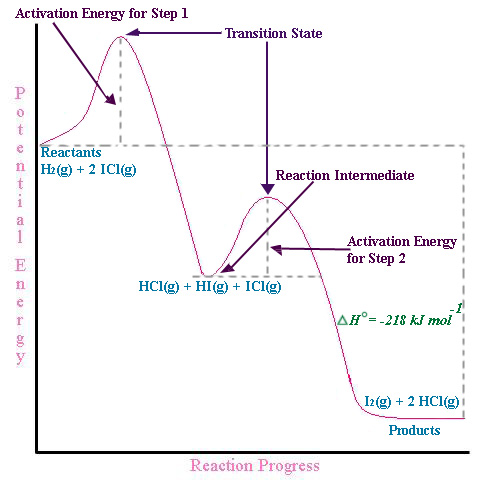
Energy Diagram for a Two-Step Reaction Mechanism A Two-Step Reaction Mechanism. The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall. Step 1 has the higher transition energy state, thus it ...
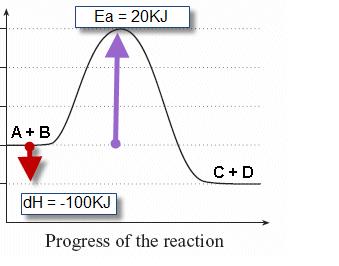
ENERGY PROFILES FOR SIMPLE REACTIONS - chemguide This diagram shows that, overall, the reaction is exothermic. The products have a lower energy than the reactants, and so energy is released when the reaction happens. It also shows that the molecules have to possess enough energy (called activation energy) to get the reactants over what we think of as the "activation energy barrier".
Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1
Energy Diagrams: Describing Chemical Reactions Some reactions occur via several steps, in any conceivable combination of the basic four shown above. Regardless of how many steps there are, though, certain facts are always true: The number of transition states = the number of steps The number of intermediates = one less than the number of steps DG rxn = G final - G initial = -RT (lnK eq )
18.5 Multi step Reaction - CK-12 23 Feb 2012 — Draw the potential energy diagram for the following multi-step reaction \begin{align*}(\triangle H < 0)\end{align*}. Properly label the ...
Answered: Reaction progress Potential energy | bartleby The following graph shows two different reaction pathways. for the same overall reaction at the same temperature. Is each of the following statements true or false? (a) The rate is faster for the red path than for the blue. path. (b) For both paths, the rate of the reverse reaction. is slower than the rate of the forward reaction. (c) The ...
Answered: 2. Show the Atomic and molecular… | bartleby Question. 2. Transcribed Image Text: 2. Show the Atomic and molecular orbitals of acetic acid. Draw the energy diagram for the AO and MO. Label the HOMO and LUMO in the energy diagram. and explain in step by step process 0=0 HO HICII -H (you might need to rotate the image to show it standing up instead of sideways)
Energy Diagrams of Reactions | Fiveable Nov 23, 2021 · Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings.
Cellular Respiration Equation, Types, Stages, Products & Diagrams Cellular Respiration Equation: C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + 38*ATP. 10. Cellular Respiration Equation: Every machine needs specific parts and fuel in order to function. Likewise, " biological machines " also require well engineered parts and good energy source in order to work. Perhaps the second most important molecule (DNA is the ...
Solved Consider the following reaction energy diagram: How - Chegg Science; Chemistry; Chemistry questions and answers; Consider the following reaction energy diagram: How many elementary steps are in the reaction mechanism?
PDF Rates, Temperature and Potential Energy Diagrams Worksheet a) Draw a potential energy diagram for this reversible reaction. Your starting value for the reactants might be different that is ok, as long as you show the proper E a and ∆H values. b) Calculate the enthalpy change (∆H) for each reaction. i) ΔH forward = 137 kJ/mol i) ΔH reverse = 137 kJ/mol 6.
How does the energy level diagram show this reaction is exothermic? Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
Solved Label the following reaction energy diagram for a - Chegg Question: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Transition State Transition State Transition intermediate Reaction Intermediate Transition State Transition Uncatalyzed Transition State Reactants Products Catalyzed Ea (fwd) no catalyst Uncatalyzed AHxn < 0 Potential energy Ea (rev) no catalyst Ea ...
Solved Label the following multi-step reaction energy | Chegg.com See the answer See the answer done loading. Label the following multi-step reaction diagram. Show transcribed image text.








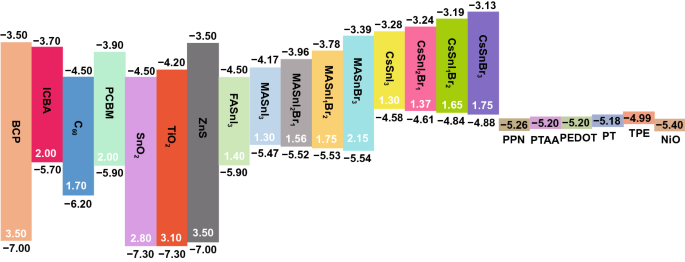


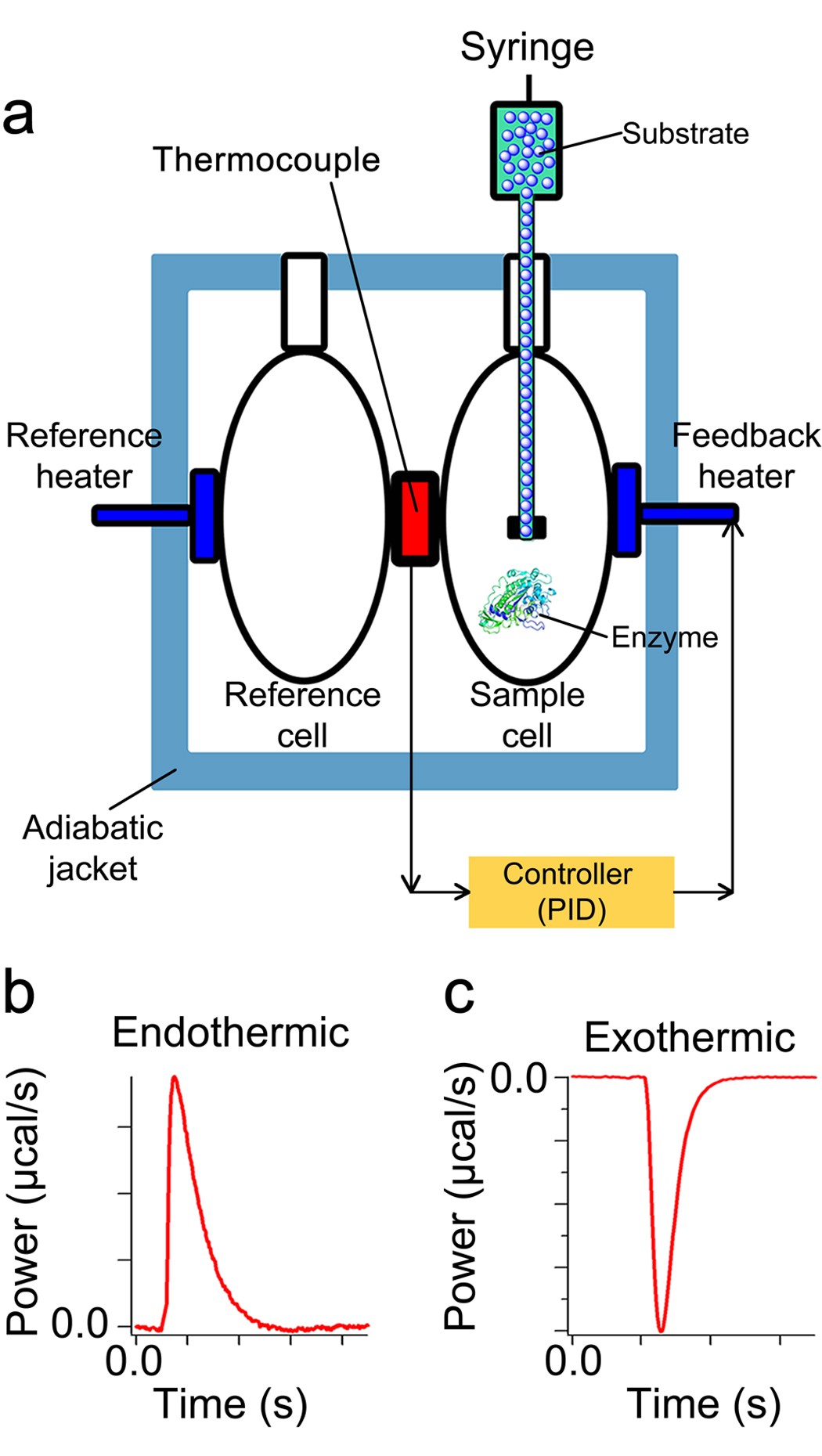
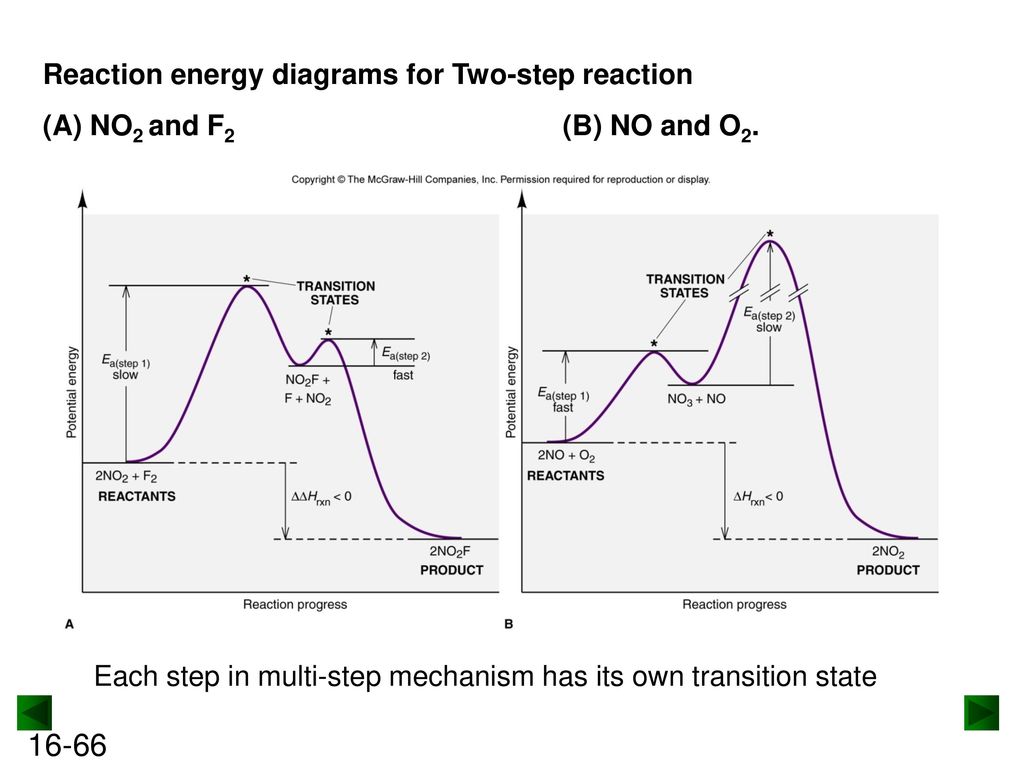

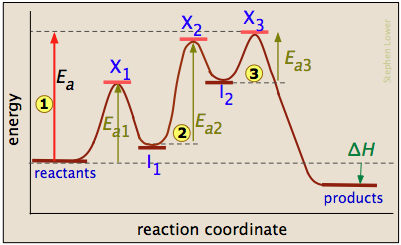


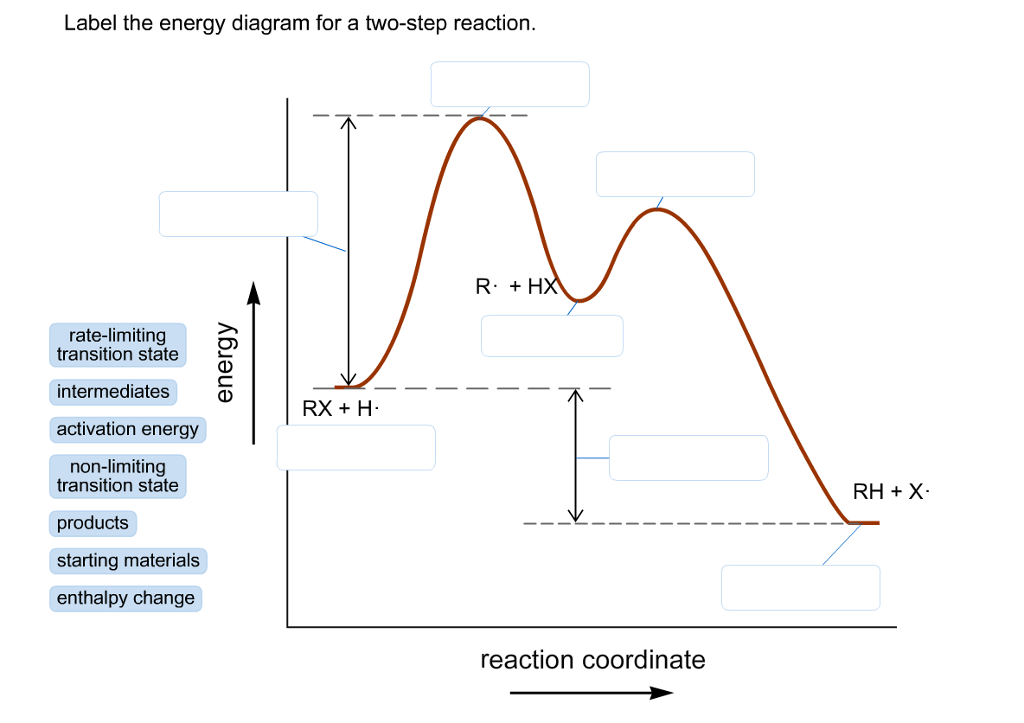









Post a Comment for "41 label the following multi-step reaction energy diagram"