44 usda generic label approval form
Prior Label Approval System: Expansion of Generic Label Approval Such labels are not submitted to FSIS, because they are deemed approved and may be applied to product in commerce. Generic label approval has been in place in some form since 1983. FSIS has previously expanded the categories of labeling claims eligible for generic approval, most recently in 2013 ( 78 FR 66826, November 7, 2013). PDF Shell Egg Label Approval - Agricultural Marketing Service Labels bearing a USDA approval number are to be initialed and dated by the grader. The actual label or a photocopy of the label and a copy of the issued Form PY-221, Label Notice, are to be placed in the grader's file (File Folder # 14). The USDA grader must review any provisions listed on the Form
Forms and Guidelines | USDA Form 6500-46 - Forest Service Authorization for In-Service Expenditures (PDF, 26.6 KB) GPO 907 Non-Compliance/Change Report (PDF, 33.9 KB) GPO 952 Desktop-Disk Information (PDF, 204 KB) GPO 1815 Notice of Quality Defects (PDF, 211 KB) GPO 3868 Notification of Intent To Publish (PDF, 115 KB) USDA Style Guide.

Usda generic label approval form
PDF Transmittal of Labeling or Outlines - Usda This form is intended as a cover page for paper submissions of labeling materials, an Outline of Production, or a Special Outline. It is not needed for submissions via the NCAH Portal. Submit one copy of this form for each paper Outline, Special Outline, or group of labeling intended for one product. Use separate forms for Outlines and labeling. eForms Home - USDA Welcome to the USDA Service Center Agencies eForms. eForms allows you to search for and complete forms requesting services from Farm Service Agency (FSA), Natural Conservation Service (NRCS), and Rural Development (RD). There are 2 ways to use the eForms site. You can click the Browse Forms menu option on the left of the page and search for ... Food Labeling - USDA The Food Safety and Inspection Service (FSIS), a public health agency within the USDA, is responsible for ensuring that the nation's commercial supply of meat, poultry, and egg products is safe and correctly labeled and packaged. FSIS issues policy guidance and information, memorandums, and nutrition labeling information.
Usda generic label approval form. FSIS Labeling Overview and Generic Label Approval | Food ... Aug 24, 2021 · The guidance guideline provides information about Agency procedures for labeling and label approval procedures for meat and poultry products to assure the products are safe and suitable. This guidance also provides additional instruction on required labeling features, new generic labeling regulations, sample labels, label submission, and ... Guidance for Industry - Food and Drug Administration The regulations require that the content of labeling be submitted in a form that we (FDA) can process, review, and archive. Since 1999, FDA has been receiving the electronic content of PDF LABELING POULTRY PRODUCTS Procedures I. Labeling A. Approval (9 C.F.R. Part 381). Labeling is the responsibility of FSIS and questions regarding the accuracy and use of any labeling bearing the official grademark should be handled through the Inspector-In-Charge (IIC). I. Labeling . A. Approval . FSIS regulations permit companies to produce generic labels without submission and to CDER Office of Compliance - Food and Drug Administration 9 . Foreign Firm Registration & Drug Listing [FDCA Sec. 510 & 21 CFR 207] All foreign firms that manufacture, prepare, propagate, compound, or process a drug imported or
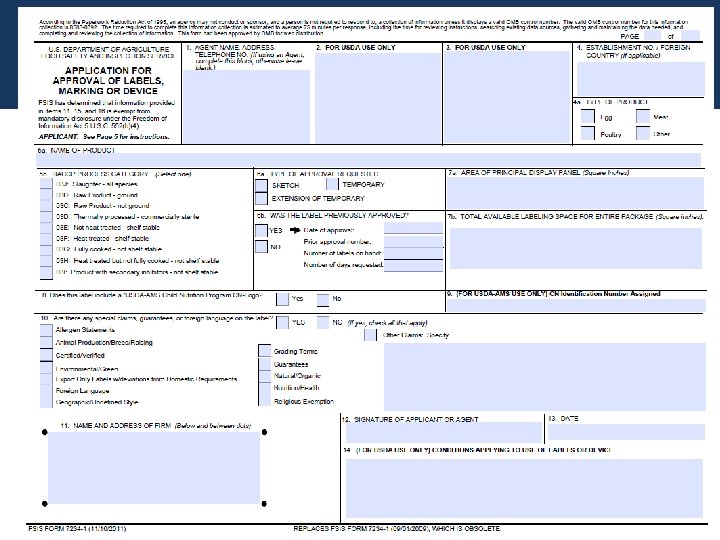
Prime Label Consultants PLC's Regulatory Consultants review both FDA & USDA labels and boast a 95% approval rate at the USDA (compared with a 63% industry average). We are available by phone, webinar, or on-site for label development, review, approval, consulting & auditing solutions. Get Started Now! PDF FSIS 7234-1 Application for Approval of Labels, Marking or Device The following instructions relate to numbered items on form. 1. If using an Agent, provide the company name, address, and telephone number, otherwise leave blank. 2 & 3. Leave blank, for USDA use only. 4. Establishment No./Foreign Country (if applicable) - Self Explanatory. 5a. Name of Product. Forms & Submission Requirements | FDA - U.S. Food and Drug Administration IND Forms and Instructions. New Drug Applications (NDAs) NDA Forms and Electronic Submissions . Abbreviated New Drug Application (ANDA) ANDA Forms and Submission Requirements. Drug Master Files ... Generic Drug Labeling: Strategies for Providing High-Quality Submissions ANDA Approvalissued early October 2015 -Provides for the labeling content of ANDAs to be approved in draft as long as any outstanding labeling deficiencies are minor and editorial in nature....
USDA/USDC Authorized Labels and Manufacturers USDA/USDC Authorized Labels and Manufacturers Home Child Nutrition Programs CN numbers that appear on the valid list apply to the CN logo and crediting statement only. It is the manufacturer's responsibility to ensure that the product label meets all over federal labeling requirements. CN Label Verification Report (8/10/2022) Label Submission and Approval System (LSAS) | Food Safety and ... Mar 08, 2022 · Guidance document for submitting an FSIS Enrollment Request for the Label Submission and Approval System (LSAS) USDA eAuthentication System "eAuth" is the secure system that allows web-based access to USDA applications and services. This USDA site tells you how to obtain a Level 2 USDA eAuthentication account. Register for an eAuth Account askFSIS Public Q&As: FSIS Labeling Records - USDA The labeling record must include: the final label applied to the product, product formulation, processing procedures and supporting documentation, including prior sketch approval from LPDS (if applicable). The recordkeeping requirements for labeling records are found in 9 CFR 320.1 (b) (10) and 381.175 (b) (6). Forms | FDA - U.S. Food and Drug Administration Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Use the following instructions to download the form if you encounter an issue:...
Prior Label Approval System: Generic Label Approval - Federal Register Generic label approval refers to the prior approval of labels or modifications to labels by the Agency without submitting such labels to FSIS for sketch approval. Generic label approval requires that all mandatory label features be in conformance with FSIS regulations ( 9 CFR 317.5 (a) (1) and 381.133 (a) (1) ).
Forms | Food Safety and Inspection Service - USDA 3800-2. FSIS Form 3800-2 Annual Notice to High Mileage Driver. 4339-1. FSIS Form 4339-1 Certificate of Medical Examination (With Report of Medical History) 4791-36. FSIS Form 4791-36 Firearm Discharge Warning System. 9060-5. FSIS Form 9060-5 Meat and Poultry Export Certificate of Wholesomeness (Completion Instructions) 9060-5.
Home | NASS Welcome to the USDA National Agricultural Statistics Service Respondent Portal. Enter your Unique Survey Code. Submit & Get Survey(s) Need Help? Call 1-888-424-7828.
PDF FSIS Compliance Guidance for Label Approval - Food Safety and ... On November 7, 2013, the Food Safety and Inspection Service (FSIS) amended its prior label approval regulations to expand the circumstances in which certain types of labels are generically approved. The Labeling and Program Delivery Staff (LPDS) evaluates four categories of labels 9 CFR 412.1 (c): labels for religious exempt products 9 CFR
FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...
USDA APHIS | APHIS Forms To view some of these forms, you will need to have the free Adobe Acrobat Reader ... 03/2006. PDF. APHIS 14-R. Yes. FTS 2001 Calling Card Tracking Sheet Generic Cards Without Names. 10/2004. PDF. APHIS 16-R. Yes. Review and Approval of Document. 01/2003. Employees Only ... Transmittal of Labeling or Outlines. 08/2017. PDF. APHIS 2017. Yes ...
USDA APHIS | Veterinary Biologics Biologics Forms. Other Biologics Activities The Center for Veterinary Biologics participates in national and international animal health and regulatory organizations. Read more about CVB activities. Biologics Links This section contains links to other sites associated with biologics regulation and international harmonization.
CN Labeling Program | Food and Nutrition Service - USDA The USDA, Child Nutrition (CN) Labeling Program provides food manufacturers the option to include a standardized food crediting statement on their product label. Labels must be authorized by USDA, FNS prior to use and manufacturers must have quality control procedures and inspection oversight that meet the FNS requirements.
Food Manufacturers/Industry | Food and Nutrition Service - USDA Dec 10, 2010 · How to Complete the 7234 Form ; Manufacturer's Product Formulation Statement (PFS) * To gain full function of the templates, either right click on the web link and save the file on your computer or open the web link, save the file on your computer, and then reopen it. Please contact cnpntab@usda.gov for further assistance.
Guidelines: Animal Products That Do Not Require An Import Permit - USDA A USDA APHIS VS import permit (VS Form 16-6), will not be required for FDA approved human pharmaceuticals, approved active pharmaceutical ingredient (usually shipped in bulk), over- the-counter (OTC) drug monographs, human vaccines, human medical devices, veterinary pharmaceuticals, and FDA regulated veterinary in bulk and/or packaged and ready ...
Generic Label Approval | Food Safety and Inspection Service This guidance provides information about procedures for obtaining prior approval for sketch and generic labels for meat and poultry products. It applies to food manufacturers and official establishments seeking to request label consideration and the actions they may take before products may be marketed. It relates to 9 CFR Part 412.
Logo | USDA The USDA logo was created and approved for use in 1995. It is the official and sole identifying mark for the Department, its mission areas, and all agency programs. The logo is the single, most visible asset of our organization. ... Use the Signature Iso-Bar on all multimedia content, interfaces, applications (apps), labeling, and packaging.
Food Labeling - USDA The Food Safety and Inspection Service (FSIS), a public health agency within the USDA, is responsible for ensuring that the nation's commercial supply of meat, poultry, and egg products is safe and correctly labeled and packaged. FSIS issues policy guidance and information, memorandums, and nutrition labeling information.
eForms Home - USDA Welcome to the USDA Service Center Agencies eForms. eForms allows you to search for and complete forms requesting services from Farm Service Agency (FSA), Natural Conservation Service (NRCS), and Rural Development (RD). There are 2 ways to use the eForms site. You can click the Browse Forms menu option on the left of the page and search for ...
PDF Transmittal of Labeling or Outlines - Usda This form is intended as a cover page for paper submissions of labeling materials, an Outline of Production, or a Special Outline. It is not needed for submissions via the NCAH Portal. Submit one copy of this form for each paper Outline, Special Outline, or group of labeling intended for one product. Use separate forms for Outlines and labeling.





















Post a Comment for "44 usda generic label approval form"