44 label all bonds in ch2br2
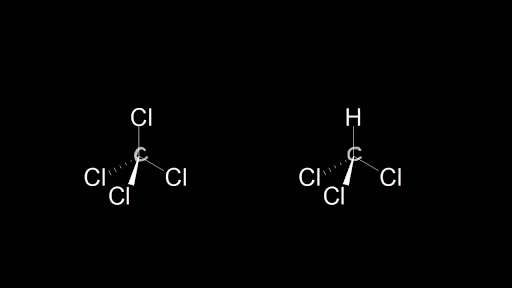
Solved Part B Label all bonds in CH2Br2 Label the diagram by | Chegg.com Transcribed image text: Part B Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used H (p) H (p) Br (o) Br (p) Previous question Next question Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in NF3. Label all bonds in BF3. Answer The molecule's name is CH2Br Br Br br dibromomethane. The molecule can be described as a derivative methane. The central atom of carbon is bonded with two hydrogen atoms, and two bromine. All bonds are sigma.
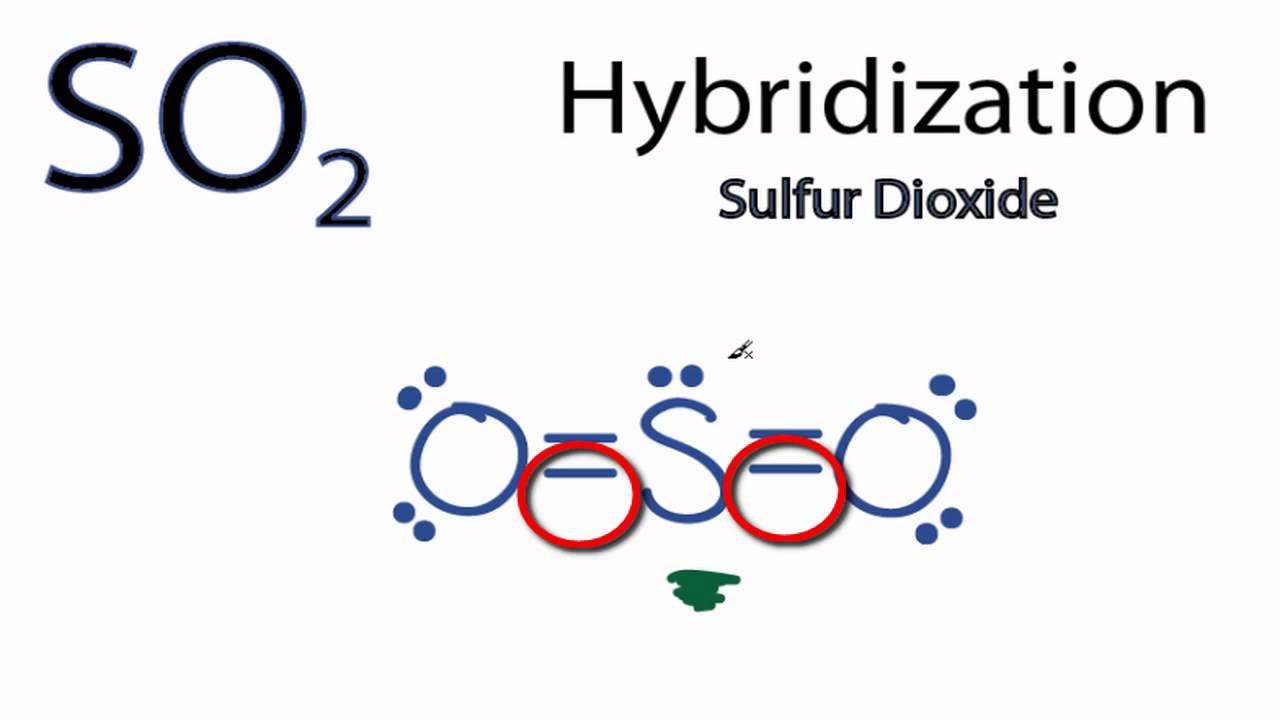
Answered: In the sketch of the structure of SO2… | bartleby In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. : S (p) - O (p) Lone pair in p orbital Lone pair in sp? orbital o : S (p) - O (sp²) т: S (p) — О (p) т: S (sp?)
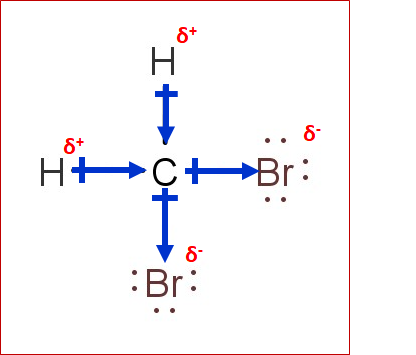
Label all bonds in ch2br2
Chemistry: Semester 2, Unit 1 Practice Problems - Quizlet 65: Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds including the notation shown in examples 10.6 and 10.7. a. N2H2 (skeletal structure HNNH) OneClass: Label all bonds in CH2Br2? Get the detailed answer: Label all bonds in CH2Br2? ... Label all bonds in CH 2 Br 2? Answer +20. Watch. 1. answer. 2. watching. 661. views. For unlimited access to Homework Help, a Homework+ subscription is required. Kottherva Sreevidya Lv10. 5 Jan 2021. Unlock all answers. Get 1 ... Chapter 10: Chemical Bonding II: Molecular Shapes, Valence Bond Theory ... VSEPR Theory. Combines the Lewis Model with the idea that valence electron groups repel one another (Covalent Bonds) to predict the general shape of a molecule from its Lewis Structure. Repulsions between electron groups on the interior atoms of a molecule determine the geometry of the molecule, and the preferred geometry is the one in which ...
Label all bonds in ch2br2. Solved Label all bonds in CH2Br2. Label all bonds in | Chegg.com Best Answer 92% (63 ratings) Transcribed image text: Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Previous question Next question CHEM: Chapter 10 Flashcards | Quizlet The sp3 and sp3d2 hybridization schemes have no unhybridized p-orbitals left to form π-bonds. Valence Bond Theory 62. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3 Solved In the sketch of the structure of CH Br2 label all - Chegg In the sketch of the structure of CH Br2 label all bonds. Drag the appropriate labels to their respective targets. (Solved) - Label all bonds in CH2Br2 Label the diagram by dragging the ... The molecule is CH2Br2. Br ...
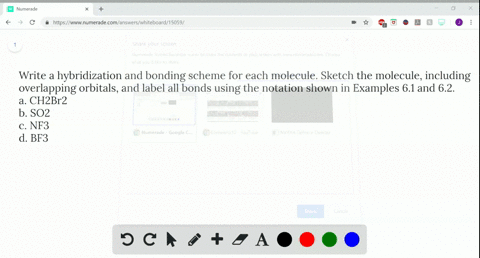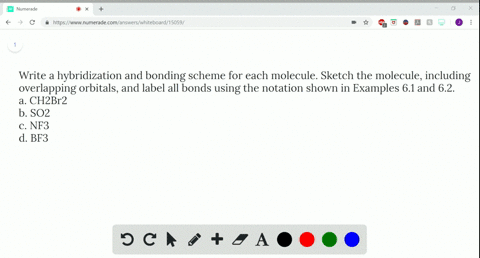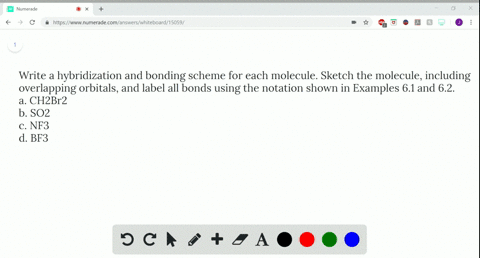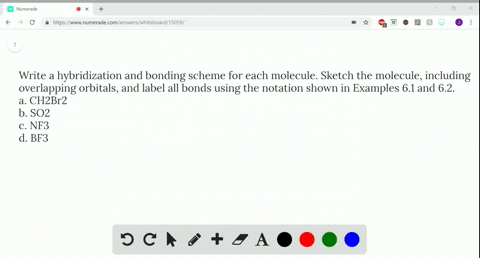
Use valence bond theory to write the hybridization and ... - Socratic Warning! Long Answer. Here's what I get. > Step 1. Draw the Lewis structure (a) Start with a skeleton structure. The two "C" atoms (least electronegative) will be the central atoms, with the "N" attached to one of the carbons. (b) Attach the hydrogen atoms. The question gives you a clue where they go. The formula "NCCH"_3 tells you that the three "H" atoms are attached to the terminal carbon atom. SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Problem 34 Hard Difficulty Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3 Answer (a) See solution (b) See solution (c) See solution (d) See solution View Answer Discussion Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in BF3. Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Expert's Answer Solution.pdf Next Previous CH2Br2 Molecular Geometry - Science Education and Tutorials The formula of CH2Br2 molecular hybridization is as follows: No. Hyb of CH2Br2= N.A (C-H and C-Br bonds) + L.P (C) No. Hy of CH2Br2= the number of hybridizations of CH2Br2 Number of C-H and C-Br bonds = N.A (C-Br and C-H bonds) Lone pair on the central carbon atom = L.P (C) Calculation for hybridization number for CH2Br2 molecule
Chapter 6, Chemical Bonding II Video Solutions, Chemistry: Structure ... Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. N2H2 (skeletal structure HNNH) b. Answered: Write a hybridization and bonding… | bartleby Science Chemistry Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3 Write a hybridization and bonding scheme for each molecule. CHEM 1210 Flashcards | Quizlet On your paper or tablet, draw a Lewis structure for this compound in the which the formal charge for each atom is zero. Include in your structure any unshared electron pairs. Identify in the boxes below the number of valence electrons, sigma bonds, pi bonds, and unshared electron pairs. valence electrons: sigma bonds: pi bonds unshared electron ... Identify The C The Of Atom In Ch2br2 Hybridization [NQRF1B] 55 Identify the hybridization state and geometry of each carbon atom in the following compounds: Predict the bond angles for all bonds For (a), draw the molecular orbitals An electron from the 22 orbital and three other electrons from 2p orbitals participate in forming bonds An electron from the 22 orbital and three other electrons from 2p ...
Answered: In the sketch of the structure of BF3… | bartleby Transcribed Image Text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Lone pair in sp orbital : B (p) - F (p) Empty p orbital Lone pair in p orbital B B (sp²) - F (p) в : В (8) — F (p) o : B (p) - F (p) Empty sp ...
Answered: Rank the free radicals (1-LII) shown… | bartleby Q: In the sketch of the structure of CH2B12 label all bonds. Drag the appropriate labels to their… A: All the bonds formed in CH2Br2 are sigma bonds. The hybridisation of C is sp3 Both, the C-Br bonds…
Drag the labels to identify the appropriate reagents for each reaction ... Each label is associated… The following solution is suggested to handle the subject "Drag the labels to identify the appropriate reagents for each reaction below. Each label is associated…". Let's keep an eye on the content below! Question "Drag the labels to identify the appropriate reagents for each reaction below.
Answered: In the sketch of the structure of… | bartleby Transcribed Image Text: In the sketch of the structure of CH2Br2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.
Identify the hybridization of the c atom in ch2br2. - Brainly.com The hybridization of the C atom in CH₂Br₂ is sp3. When bonding, the orbitals "s" and "p" from C atoms interact to form hybridized orbitals. If the C atom has 4 sigma bonds, as is the case in CH₂Br₂, there are 4 hybridized orbitals required, so 1 "s" orbital and 3 "p" orbitals hybridize to form an sp3 hybrid orbital.
Label each molecule with the name of its shape. - Brainly.com In , iodine has seven valence electrons it utilizes five of these to form bonds with five fluorine atoms. The remaining two electrons are accommodated as the lone pair. In order to have minimum repulsion as per the VSPER theory this fourth pair must lie in the plane above the four I-F bonds, and this constitutes the square pyramidal shape.
How many bonds are in CH2Br2? - Answers Does CH2Br2 form hydrogen bonds? No, hydrogen bonding only occurs in compounds where hydrogen (H) is bonded to nitrogen (N), oxygen (O) or fluorine (F). How many isomers of ch2br2? This compound...
Chapter 10: Chemical Bonding II: Molecular Shapes, Valence Bond Theory ... VSEPR Theory. Combines the Lewis Model with the idea that valence electron groups repel one another (Covalent Bonds) to predict the general shape of a molecule from its Lewis Structure. Repulsions between electron groups on the interior atoms of a molecule determine the geometry of the molecule, and the preferred geometry is the one in which ...
OneClass: Label all bonds in CH2Br2? Get the detailed answer: Label all bonds in CH2Br2? ... Label all bonds in CH 2 Br 2? Answer +20. Watch. 1. answer. 2. watching. 661. views. For unlimited access to Homework Help, a Homework+ subscription is required. Kottherva Sreevidya Lv10. 5 Jan 2021. Unlock all answers. Get 1 ...
Chemistry: Semester 2, Unit 1 Practice Problems - Quizlet 65: Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds including the notation shown in examples 10.6 and 10.7. a. N2H2 (skeletal structure HNNH)
















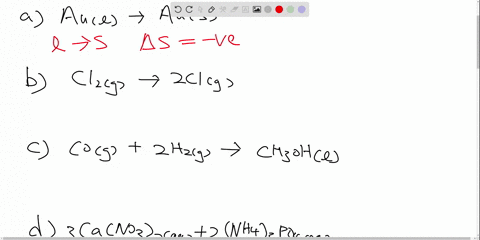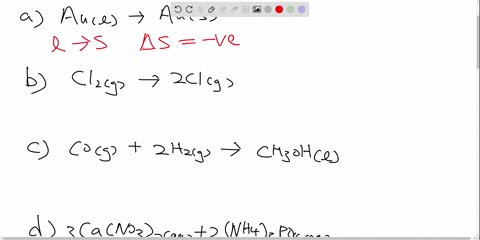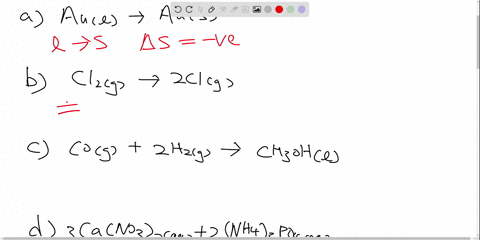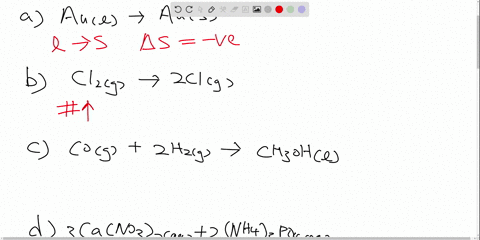

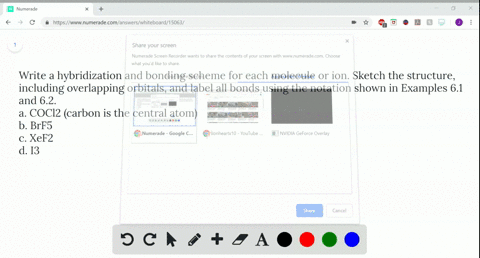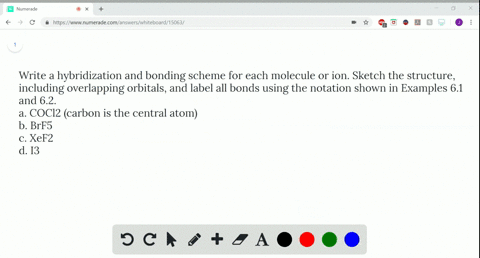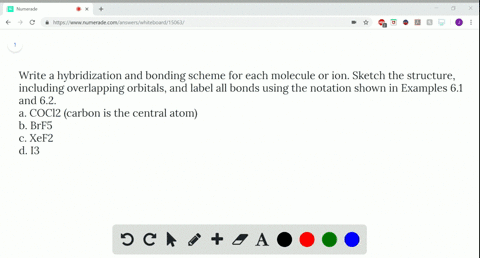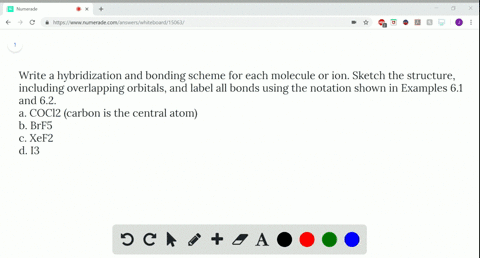





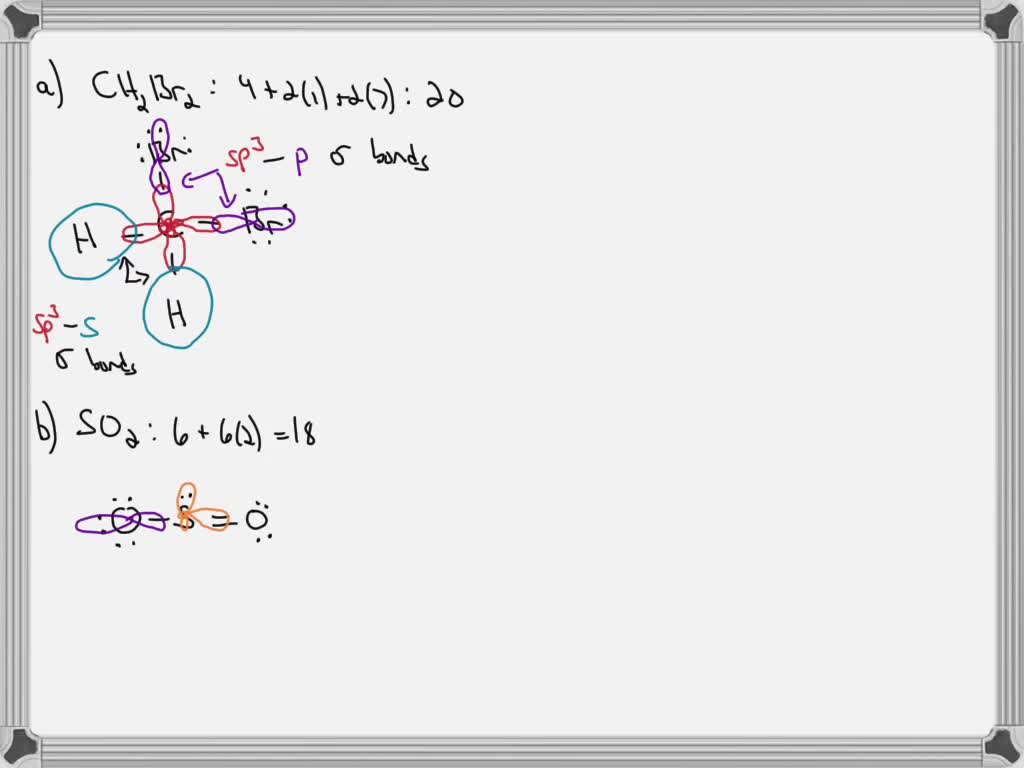


Post a Comment for "44 label all bonds in ch2br2"